What Came First: Thermodynamics or the Steam Engine?
There is a short answer and a long answer to this question. The short answer is simple: the steam engine predates thermodynamics. But the long answer is much more interesting when we consider the cultural and industrial variables at play during the birth of energy science.
Simply, the steam engine predates thermodynamics by over a century. In fact, its practical and industrial applications drove the development of what became thermodynamics in the mid-nineteenth century.
The implications of this point are worth unpacking. We are somewhat used to thinking about theoretical science as the driver of experimental and applied sciences. That is, we might be tempted to believe that scientists develop a theory and then apply it to produce new technologies. The history of science shows us that this is not always how science operates. In the case of thermodynamics, theoretical physics certainly did not steer the course of what became the science of energy physics. Industry did.
This post provides a brief overview of the development of the British steam engine, and how the science of thermodynamics emerged from the problems produced by industrial shifts. Most importantly, though, I explain how these laws were not just discovered, but rather addressed specific worldviews, technological complications, and material questions tied to commerce, imperialism, and the ideal of progress. This is important to understand because such ideals set the tone for writing energy into scientific natural law, and for how, almost two hundred years later, we continue to imagine energy and its possibilities.
The Invention of the Steam Engine
The Victorian period was the age of steam power. It was the steam engine that released textile mills and other industry from water power’s geography-dependent architectures, driving populations to new, smoky factory cities. But this iconic steam imaginary owed nothing to thermodynamics at the outset. In fact, it was the other way around.
Historians trace modern mass politics and ways of living to industrial organizations of fossil fuel energy [1]. England burned coal as early as the thirteenth century, yet it wasn’t until what we call the “Industrial Revolution” (the mid-eighteenth century to the mid-nineteenth century) that humans transitioned to a coal-based energy system. Until then, steam engines consumed more fuel than they could extract from England’s water-filled coal mines. After improved engines abetted mining and industrial iron production, England began to harness its network of waterways to cheaply transport coal. This self-reinforcing system of geography and industry is why, rather than superior technology or innovative ability, Britain’s development “diverged” from other parts of the world such as China, Japan, and India [2].
As early as the 1690s, inventors played around with steam-pressured pumps. Thomas Savery patented his “engine to raise water by fire” during this period and published an accompanying text, The Miner’s Friend, in 1702 [3].
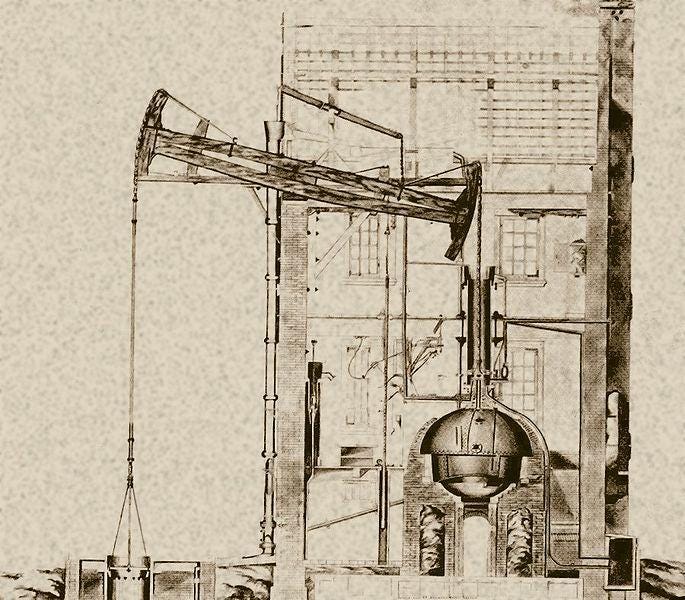
Savery’s steam pump was not particularly useful for coal extraction, however. It was Thomas Newcomen (1663-1729) whose atmospheric steam engine was effective enough to address the demand of Cornish miners [4]. Newcomen’s engine worked by letting low-pressure steam move from a boiler into a cylinder directly above it. As soon as the piston reached the top of this cylinder, the engine operator sprayed cold water directly into the cylinder, condensing the steam. By doing this, the operator created a partial vacuum which allowed the atmospheric pressure to push the piston downward.
Understanding how the Newcomen engine works helps us appreciate James Watt’s contribution to this history.
But wait! Didn’t James Watt invent the steam engine? Obviously no, he did not; though “inventor of the steam engine” is a title that histories do sometimes bestow upon Watt. It was Watt, however, who separated the boiler and condenser chambers in his engine, and that made a huge difference in engine efficiency. Additionally, Watt built a partnership with Matthew Boulton, who we can think of as the commercial mastermind of the steam engine. Boulton found a market beyond Cornwall miners: the factory. He realized that mill owners were desperate to add energy-saving technologies to their machinery, and Watt’s engine delivered [4]. By the early 1800s, inventors were beginning to experiment with other energy-saving tweaks, which brings us back to the question of thermodynamics.
Sadi Carnot and the First Stirrings of Thermodynamics
If you’ve studied thermodynamics or taken a heat transfer course, you likely learned about the “Carnot cycle.” In this classic model, a system moves through a series of what are called adiabatic and isothermal stages, returning to its original state in the completion of one “cycle.” Thus, the cycle is theoretically reversible, performing mechanical work on its surroundings with maximum efficiency. It isn’t important to understand the ins and outs of the Carnot cycle for this discussion, but it might be helpful to see it represented graphically. We calculate the mechanical work of the Carnot cycle by measuring the area enclosed in the curve created by the changes in volume and pressure.

What you may not know is that the Carnot engine cycle nearly slipped into obscurity after Sadi Carnot’s early death; and, were his writings not excavated by a few key individuals, thermodynamics may have failed to materialize under the precise conditions that it did.
Sadi Carnot was a French military scientist, following the footsteps of his father, Lazare Carnot (1753-1823), who served as one of Napoleon’s leading scientists and was forced into exile after the restoration of the French monarchy in 1815 [4]. In the summer of 1824, Sadi published a slim volume titled Reflections on the Motive Power of Fire and on Machines Fitted to Develop that Power. Few read this book during Carnot’s lifetime, and it was nearly lost forever after his death in 1832. However, a French engineer named Émile Clapeyron uncovered Carnot’s text and recast many of his verbal arguments into mathematical form in 1834. From this, the “Carnot Cycle” captured the attention of scientists trying to retool the steam engine into a more efficient machine.
One of these scientists was William Thomson (later Lord Kelvin), who combined Carnot’s and the young English physicist James Prescott Joule’s ideas to carve out what is arguably the first iteration of the laws of thermodynamics. Thomson wasn’t alone in doing this work: classical thermodynamics was a discipline built from the collaborations of many nineteenth-century scientists. But it is important to understand how and why Carnot’s insights motivated Thomson to codify thermodynamics.
What Carnot gave us was a powerful metaphor for conceptualizing heat transfer. Carnot argued that a steam engine works like a water wheel: a water wheel produces work because water falls through a height. Similarly, he said, we extract work from a steam engine because heat falls from a higher temperature to a lower one. You can maximize work extraction by raising the height that water falls. Likewise, allow heat to “fall” through a larger temperature differential and you can maximize work output [5].
Let’s take a moment to think about this revolutionary concept.
At the time of Carnot’s writing, scientists thought that heat was an invisible fluid called “caloric.” Much like water moving from one height to another, the amount of caloric was thought to remain constant as it moved from one temperature to another. In passing from the boiler to the condenser, caloric simply transferred from a concentrated to a diffuse state. It did not disappear. The water wheel metaphor tracks in this regard: we move the water from a high state to a low state, but the amount remains consistent throughout the cycle.
Despite that heat is not a substance, and despite that scientists knew this by the time Thomson pored over Carnot’s work, Carnot more or less demonstrated the principle of entropy. He argued that the amount of work a steam engine can produce is limited by the temperature differential, or the difference between its hottest and coldest temperatures. And thus, heat always flows from a hot temperature to a colder one: we can’t make it flow backwards.
The banality of a statement like this today makes us lose our respect for its revolutionary potential in a pre-thermodynamics world. Carnot was demonstrating that there is an upper limit on how efficient an engine can be, and he argued that his proposed cycle was that limit.
So, we have our answer: the steam engine came first, and it inspired the science of thermodynamics. In fact, William Thomson and his brother James were deeply wedded to Carnot’s metaphor of the waterwheel, and they even built models to study Carnot’s approach. James Thomson’s practical training in marine engineering and steam engines directed both Thomson brothers to the problem of industrial waste, or machine power consumption.
This is where the intersection of thermodynamics and steam engines is particularly important. William Thomson admired Carnot not just for his scientific insights, but because Carnot believed that with an “ideal” engine, France might efficiently “carry the fruits of civilization over portions of the globe where they would else have been wanting for years” [5]. He was a favorite among scientists and engineers who puzzled out the problem of industrial work losses because a win for industry was a win for the nation.
Carnot envisioned, for France rather than England, that revolutionizing the steam engine cycle would enable miners to release far more coal energy from the ground than an engine expended. In Carnot’s words, “The most signal service that the steam-engine has rendered to England is undoubtedly the revival of the working of the coal mines, which had declined, and threatened to cease entirely, in consequence of the continually increasing difficulty of drainage, and of raising of the coal… To take away today from England her steam-engines would be to take away at the same time her coal and iron. It would be to dry up all sources of wealth, to ruin all on which her prosperity depends, in short, to annihilate that colossal power” [5]. For Carnot, the military scientist, this was an imperial mission, and a civilizing mission. The nation with the easiest access to coal would have the strongest military, the tools to conquer and occupy remote lands, and the industrial power to funnel resources back from the periphery and commercialize them.
Of course, Britain accomplished all of that, reaching the zenith of its imperial status by the end of the nineteenth century. Consider that coal abundance in Britain freed up agricultural populations whose land had previously supplied fuel and food. Unfettered by the seasonal and geographical limitations of waterpower, industry thrived in urban locations, where populations grew dense during the remainder of the nineteenth century. As labor forces turned increasingly to producing industrial goods, Britain relied on its peripheral territories for food and raw materials. Without such uncompensated labor and lifeways, Britain could not have sustained its growth and imperial status. This is a conversation beyond the scope of the present discussion, but it is too important not to mention.
Therefore, we are left with an easy answer with far more complicated resonances. The steam engine predates thermodynamics, and therefore it was what we think of as “applied science” that precipitated the development of classical energy physics. Yet a crucial combination of imperial expansion, industrial necessity, and colonial violence produced thermodynamics, rather than the detached discoveries and observations of scientists. To my mind, the cultural forces involved in the birth of thermodynamics are some of the most important reminders that energy science always was, and remains, wedded to the ideals of extraction, accumulation, and exploitation.
Citations
[1] Mitchell, Timothy. Carbon Democracy: Political Power in the Age of Oil. Verso, 2011.
[2] Pomeranz, Kenneth. The Great Divergence: China, Europe, and the Making of the Modern World Economy. Princeton University Press, 2000.
[3] Savery, Thomas. The Miner’s Friend; or, An Engine to Raise Water by Fire. 1702. Reprinted London: W. Clawes, 1827.
[4] Hunt, Bruce J. Pursuing Power and Light: Technology and Physics from James Watt to Albert Einstein. Johns Hopkins University Press, 2010.
[5] Carnot, Sadi. Reflections on the Motive Power of Fire and on Machines Fitted to Develop that Power. 1824. Translated by R.H. Thurston. Edited by Eric Mendoza. Dover Publications, 1960.






